In the H2 A Level Chemistry syllabus, students are expected to know the details of 5 organic chemistry mechanisms in detail.
In this post, we’ll summarize these organic chemistry mechanisms.
The 5 Organic Chemistry Reaction Mechanisms in H2 A Level Syllabus
There are 5 key organic chemistry reaction mechanisms that students need to know in detail for the A Level H2 Chemistry exams. They are:
- free radical substitution
- electrophilic addition
- electrophilic substitution
- nucleophilic substitution
- nucleophilic addition
In this article, we’ll go through each of them in detail.
What are Organic Chemistry Reaction Mechanisms?
An organic chemistry reaction mechanism gives a complete overview of how the entire reaction takes place. It looks at all the steps (both slow and fast steps) needed to form the final product. In H2 A Level Chemistry, students are expected to show the flow of electrons in the mechanism.
Free Radical Substitution of Alkanes
In the H2 A Level Chemistry, students are expected to know the free radical substitution of alkanes in detail. The free radical substitution of alkanes proceed via 3 steps: initiation, propagation and termination.
Let’s use the reaction between chlorine and methane as an example to illustrate the free radical substitution of alkanes.
Free radical substitution: Chlorine and Methane in the presence of UV light
Overall reaction:

Initiation: Homolytic fission of Cl- Cl bond breaks to form chlorine free radicals

Propagation: The free radicals generated in the initiation step go on to generate other types of free radicals. This propagates the reaction further.

Termination: Free radicals collide with each other and form a covalent bond with each other.
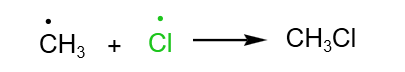


Electrophilic Addition of Alkenes
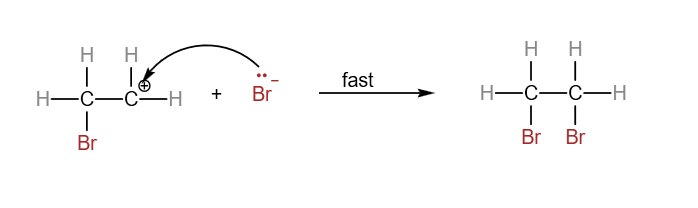
In the H2 A Level Chemistry, students are expected to know the electrophilic addition of alkenes in detail. The slow step involves the attack by electrophile to form the carbocation. The fast step involves the formation of the final product from the carbocation. There are quite a number of electrophilic addition reactions of alkenes which you can find in this post here. We’ll use the example of the reaction between ethene and bromine as an example. Electrophilic addition reaction between ethene and bromine
Overall reaction between ethene and bromine:

Step 1:
In the slow step, Br-Br approaches the electron-rich C=C. The pi electrons of the C=C interacts with bromine and causes the Br-Br bond to be polarised.
The bromine with the 𝛿+ sign attacks the electron-rich C=C to form a carbocation. Heterolytic fission of Br-Br bond occurs, forming Br–.

Step 2:
In the fast step, the carbocation and the bromide ion react to form 1,2-dibromoethane.
Electrophilic Substitution of benzene (arene)
In the H2 A Level Chemistry, students are expected to know the electrophilic substitution reaction of arenes or benzene in detail. To describe the mechanism, students would need to write 3 steps:
- Step 1: Generating the electrophile from the catalyst
- Step 2: Electrophile reacts with benzene ring to form carbocation
- Step 3: Loss of proton from carbocation to form final product.
For the electrophilic substitution reactions for arenes, the mechanism can be written using this template:
Step 1: Generating of the electrophile from catalyst.
There are quite a number of electrophilic substitution reactions that students are expected to know. You can find the list here.
| Reaction | Equation to show how electrophile (E+) is generated. |
| Nitration | HNO3 + H2SO4 ⇌ HSO4– + NO2+ + H2O Electrophile generated: NO2 + |
| Halogenation | AlCl 3 + Cl2 —> AlCl 4 – + Cl+ Electrophile generated: Cl+ |
| Alkylation | AlCl 3 + RCl —> AlCl 4 – + R+ Electrophile generated: R+ where R is an alkyl group. |
| Acylation | AlCl 3 + RCOCl —> AlCl 4 – + RCO+ Electrophile generated: RCO+ where R is an alkyl group. |
Step 2: Electrophile (E+) and benzene react to form a carbocation.
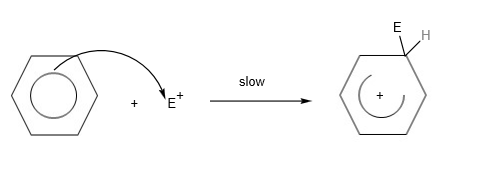
Step 3: Loss of protons from carbocation to form final product.

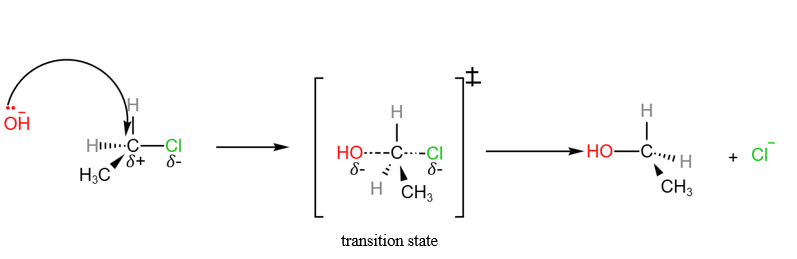
Nucleophilic Substitution of halogenoalkanes – SN1 and SN2 mechanisms
In the H2 A Level Chemistry, students are expected to know the nucleophilic substitution of halogenoalkanes in detail. There are 2 nucleophilic substitution mechanisms that students are expected to know.
- SN1 (unimolecular) : where the slow step involves only 1 molecule
- SN2 bimolecular): where the slow step involves 2 molecules
We will use the hydrolysis of halogenoalkanes to illustrate both SN1 and SN2 reactions.
SN1 Reaction between 3-chloro-3-methyl hexane and aqueous sodium hydroxide
Overall reaction:

Step 1: Formation of carbocation

Step 2: Reaction of carbocation with hydroxide ion to form alcohol.

SN2 Reaction between chloroethane and aqueous sodium hydroxide
Nucleophilic Addition of carbonyl compounds
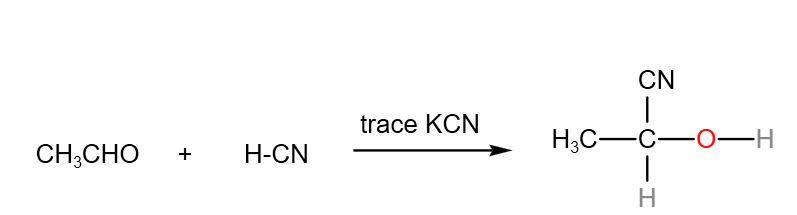
In the A level H2 Organic Chemistry syllabus, students are expected to know the nucleophilic addition of carbonyl compounds with H-CN in detail.
In this example, let’s use the reaction between HCN and ethanal with trace amount of KCN as an example to illustrate the nucleophilic addition reaction.
Overall reaction between hydrogen cyanide and ethanal with trace amount of KCN.
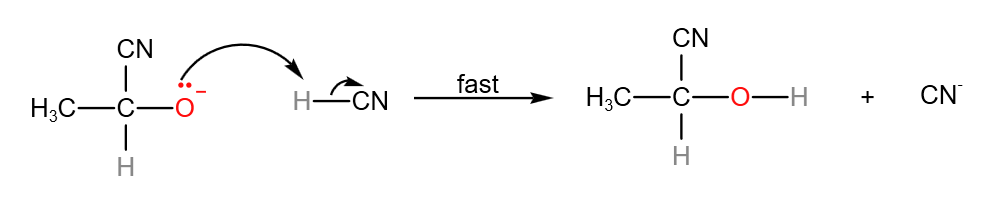
Step 1: In the slow step, the nucleophile, CN– attacks the electron deficient carbonyl carbon to form a carboanion.

Step 2: In the fast step, the carboanion gains a proton to form the product. CN– is also generated.
Applying the Organic Chemistry Reaction Mechanisms
Once you have learnt these organic reaction mechanisms in detail, the next step is to learn how to adapt them. Check out the ten- year series, there are quite a number of questions where the questions give you a reaction that is not in your syllabus, and tell you how the reaction occurs, and you are expected to draw out the mechanism. Regardless what reaction is tested, it’s basically adapting the concepts from these mechanisms that you have learnt.
Related Post
Learn H2 Chemistry On- Demand
H2 Chemistry Books
Organic Chemistry: An Essential Summary for H2 A Level Chemistry Study
Revision
Reactions
- Organic Chemistry Reactions Tested in H2 A Level Chemistry
- Organic Chemistry Reaction Mechanisms
- Organic Chemistry Concept Maps Part 1
- Organic Chemistry Concept Maps Part 2
- Structural Elucidation Questions

