Knowing the organic chemistry reactions is important when taking the A Level H2 Chemistry exam. There are just so many so many reactions to remember. Each chapter have many reactions (with the exception of alkane chapter) which students are expected to know by heart. In this post, I’ve summarized the organic chemistry reactions and listed them according to functional groups.
You can click on the link below to go directly to the reactions in the chapter you are interested in below:
- alkanes
- alkenes
- arenes
- halogenoalkanes
- alcohols
- phenols
- carbonyl compounds
- carboxylic acids and derivatives
- nitrogen compounds
Organic Chemistry Reactions: Alkanes
The functional group with the least number of reactions you need to know for H2 A Level Organic Chemistry is alkane. Basically just one reaction – free radical substitution with halogens.
- Free Radical Substitution
Alkanes react with halogens (e.g. chlorine, bromine) in the presence of uv light (or heat) to form halogenoalkanes. Multiple substitution may take place.
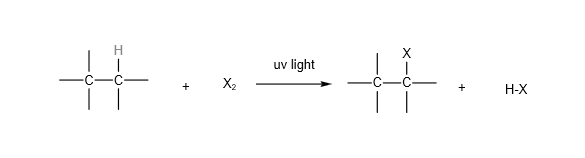
Organic Chemistry Reactions: Alkenes
- Addition Reactions
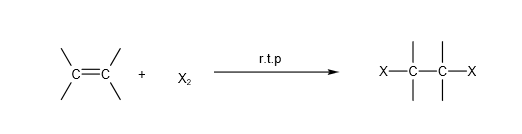
A. Electrophilic addition of halogens (X2) inert solvent (e.g. CCl4):
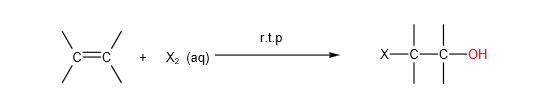
B. A. Electrophilic addition of halogens (X2) in aqueous solutions (aq):
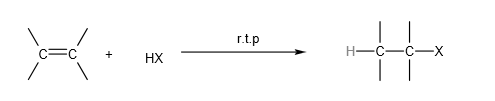
C. Electrophilic addition of hydrogen halide (HX):
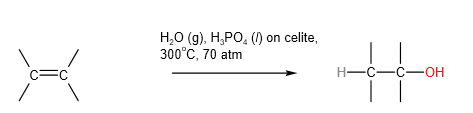
D. Hydrolysis of alkenes
Laboratory method:
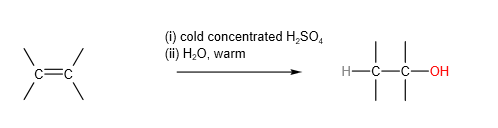
Industrial method:
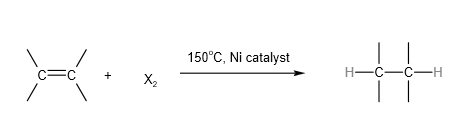
2. Reduction Reaction
3. Oxidation
A. Combustion (combustion is a type of oxidation, and during complete combustion, the most oxidised state of a compound is formed).
Alkenes undergo complete combustion to give carbon dioxide and water.
alkene + O2 —> CO2 + H2O
B. Strong Oxidation
When heated with hot acidified potassium manganate, C=C bond cleaves. Depending on what is directly bonded to each alkene carbon, a variety of products could be formed:

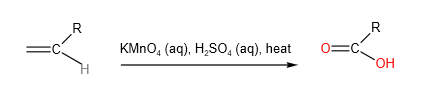
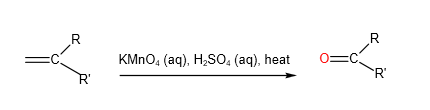
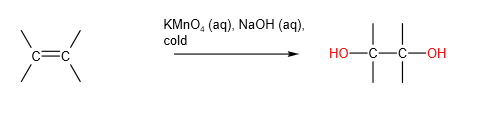
C. Mild Oxidation
Organic Chemistry Reactions: Arenes
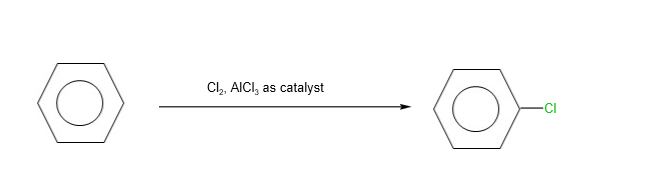
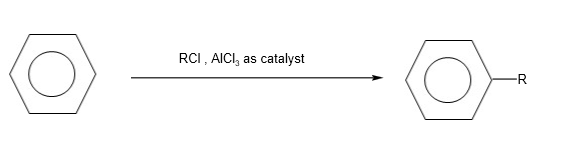
- Electrophilic substitution reactions
A. Nitration

B. Halogenation
C. Alkylation
D. Acylation

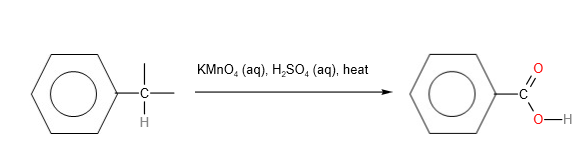
2. Oxidation of alkyl chains bonded to benzene ring
As long as the carbon directly bonded to the benzene ring is directly bonded to at least 1 hydrogen atom, the alkyl chain will be oxidised to -COOH in the presence of hot, acidified potassium manganate (VII).
Organic Chemistry Reactions: Halogenoalkanes
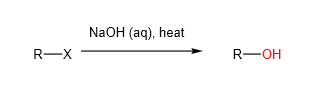
- Nucleophilic Substitution reactions:
A. Alkaline Hydrolysis
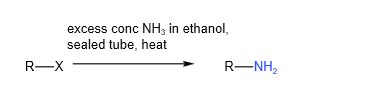
B. Reaction with ammonia
C. Reaction with KCN

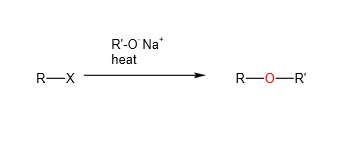
D. Reaction with sodium alkoxide
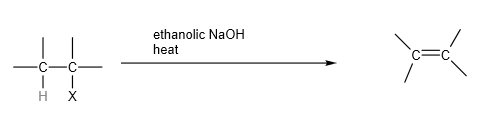
2. Elimination
Halogenoalkanes undergo elimination when heated with sodium hydroxide dissolved in alcohol
Organic Chemistry Reactions: Alcohols
- Halogenation
The -OH group in alcohols can be converted to -X group (where X is a halogen) by many different reagents which includes:
A. HX (g), heat (where X is Cl, Br or I)
B. PX3, r.t.p (where X is Cl, Br or I)
C. PCl5 (r.t.p)
D. SOCl2 (r.t.p)
Reaction: R-OH —> R-X
2. Elimination/ Dehydration
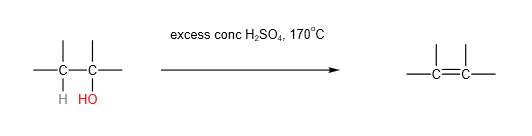
Alternatively, alkenes can also be formed by passing alcohol vapours over aluminium oxide catalyst (Al2O3) at 350 oC.
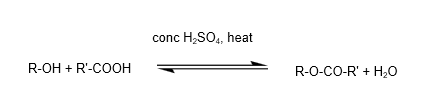
3. Condensation reaction – to form esters
A. Reaction of alcohols with carboxylic acid in the presence of concentrated sulfuric acid as catalyst, and heat, to form esters (reaction is reversible)
B. Reaction of alcohols with acyl halides at room temperature to form esters. Hydrogen halide (HX) is also produced.

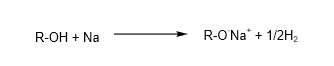
4. Reaction with reactive metals
Alcohols react with reactive metals e.g. sodium to form salt and hydrogen.
5. Oxidation
A. Oxidation of primary alcohols
Primary alcohols can be oxidised to aldehydes or carboxylic acids depending on the conditions.
Aldehydes are the “less oxidised” form compared to carboxylic acids.
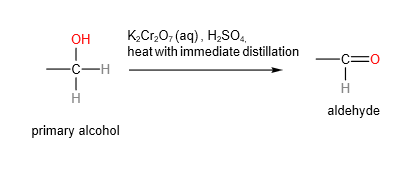
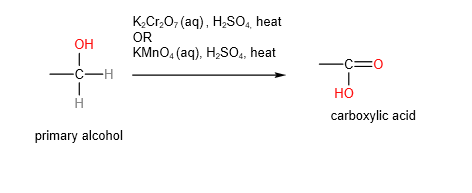
B. Secondary alcohols will be oxidised to ketones using either hot acidified potassium manganate (VII) or potassium dichromate
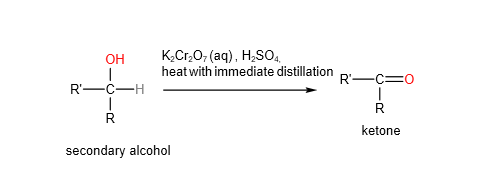
C. Tertiary alcohol will not be oxidised by hot acidified potassium manganate (VII) or potassium dichromate.
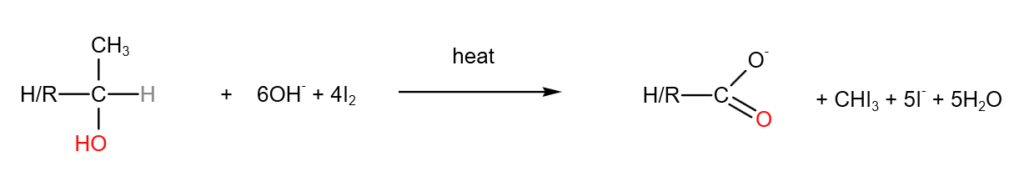
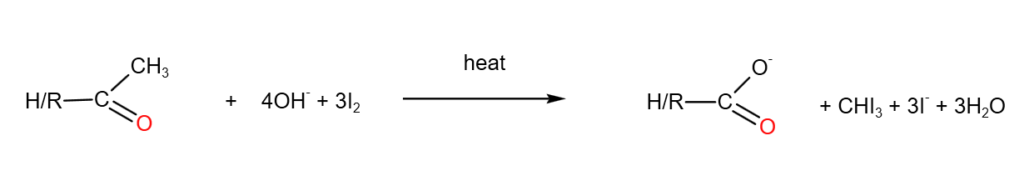
6. Iodoform test (tri-iodomethane test)
Iodoform test involves adding NaOH(aq) and I2 (aq) or alkaline iodine to a sample, and heat to test for the following structures:
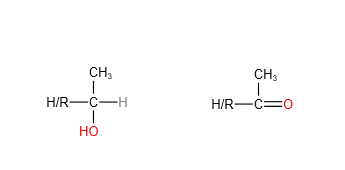
If the above structures are present, a yellow precipitate of CHI3 will be observed.
A complete balanced equations are as follows:
Organic Chemistry Reactions: Phenol
- Reaction with reactive metals
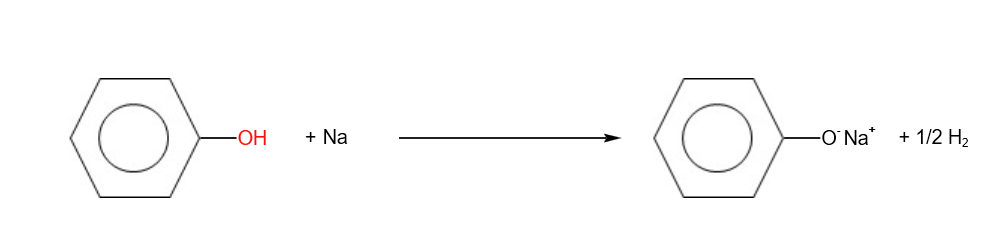
2. Reaction with alkalis (acid- base reaction)

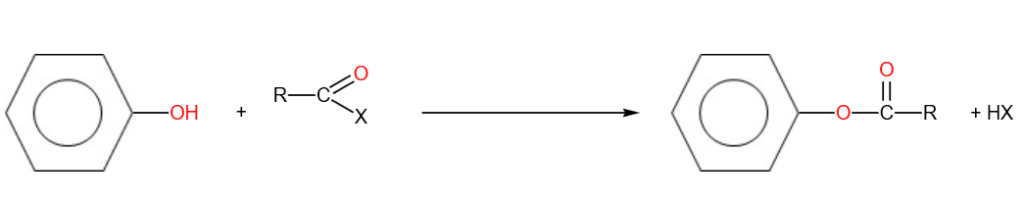
3. Reaction with acyl halides (Acylation)
Phenols react with acyl halides to form esters at room temperature. To speed up the reaction, sodium hydroxide could be added to act as a catalyst.
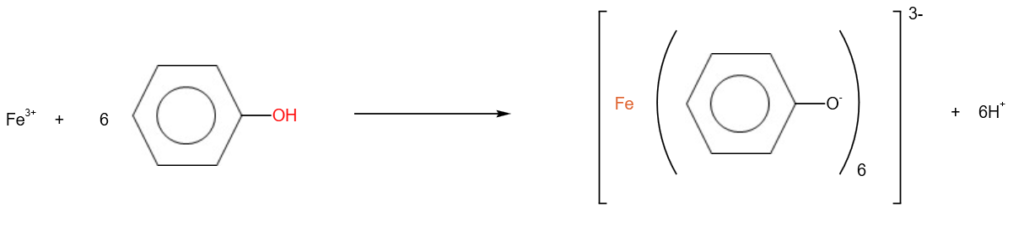
4. Complex formation with iron (III) chloride
Phenol reacts with iron(III) chloride solution to form a violet complex.
5. Electrophilic substitution of the benzene ring of phenol
In phenol, the lone pair electrons on O in -OH group delocalises into the benzene ring, making the benzene ring more electron rich and as such more susceptible to electrophilic attack.
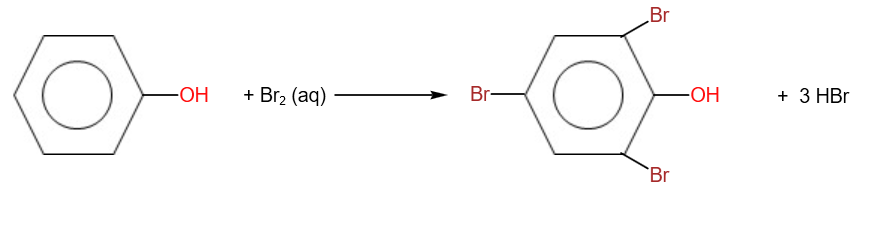
A. Halogenation:
Halogenation takes place at room temperature with the absence of a catalyst.
With aqueous halogens, tri- substitution occurs. Reddish brown bromine decolourises, and a white precipitate of 2,4,6-tribromophenol is formed.
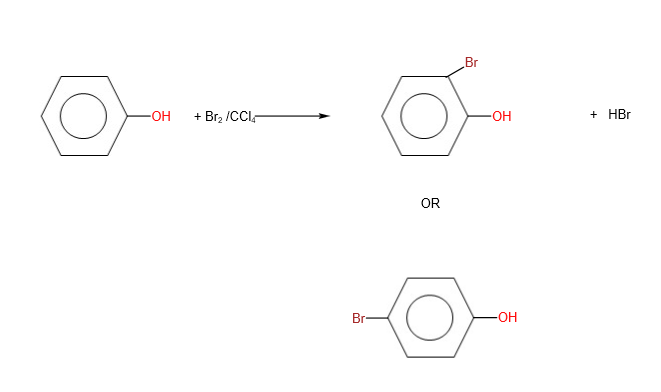
When reaction takes place with halogen dissolved in an inert solvent, electrophilic substitution takes place at room temperature with an absence of a catalyst. However, only mono- substitution occurs. As -OH group is 2-, 4- directing, the halogen goes to the 2nd or 4th position with respect to -OH.
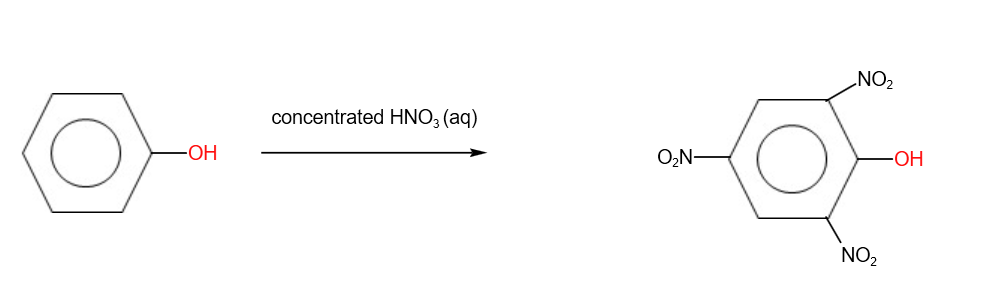
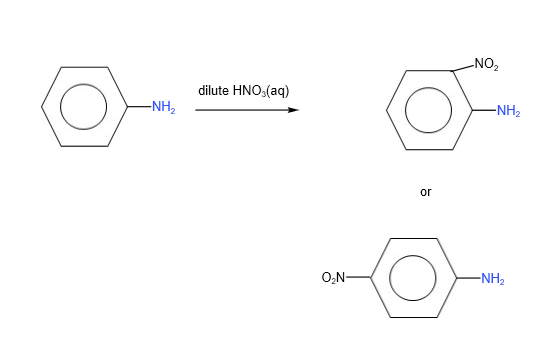
B. Nitration
With concentrated nitric acid, tri- substitution takes place.
With dilute nitric acid, mono- substitution takes place.
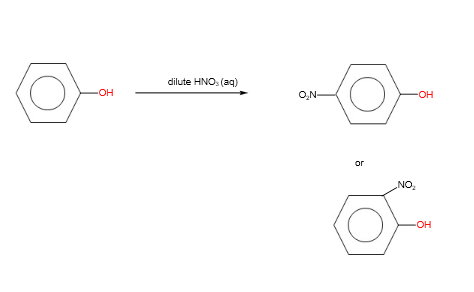
Organic Chemistry Reactions: Carbonyl Compounds
- Nucleophilic Addition
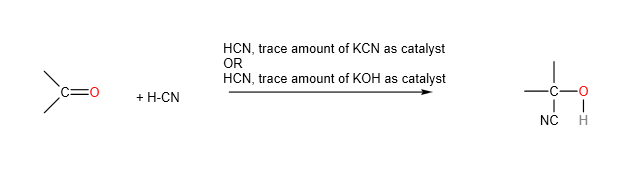
2. Condensation
Carbonyl compounds (both ketones and aldehydes) reacts with 2,4-dinitrophenylhydrazine to give an orange precipitate. This test is used to test for presence of carbonyl compounds.
Structure of 2,4- dinitrophenylhydrazine:

Reaction of carbonyl compounds with 2,4- dinitrophenylhydrazine
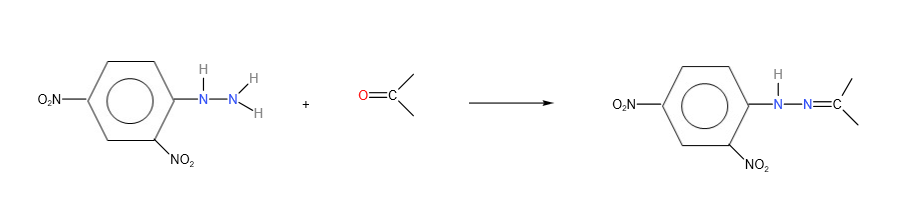
3. Reduction
Carbonyl compounds can be reduced by the following reducing agent:
- NaBH4
- LiAlH4 in dry ether
- H2, Ni catalyst, heat
Ketones can get reduced to form secondary alcohols

Aldehydes can get reduced to form primary alcohols.

4. Oxidation
A. Aldehydes undergo oxidation to form carboxylic acids using either hot acidified potassium manganate (VII) or potassium dichromate (VI)
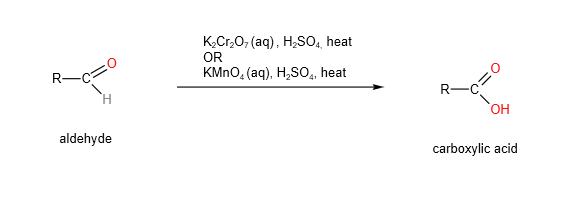
Ketones cannot be oxidised by hot acidified potassium manganate (VII) or potassium dichromate (VI).
5. Tollen’s Reagent
When Tollen’s reagent is added to aldehydes and heated, a silver mirror is formed. This test is used to distinguish aldehydes form other functional groups.
Tollen’s reagen = alkaline diamminesilver(I) = [Ag(NH3)2]+
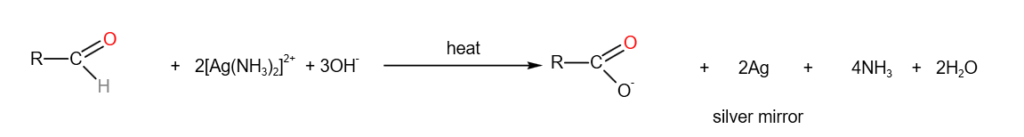
6. Fehling’s Reagent
Fehling’s reagent is used to test for aliphatic aldehydes. Aliphatic aldehydes will give a reddish-brown precipitate when heated with Fehling’s solution.
Fehling solution is a solution containing a complex ion of copper (II).
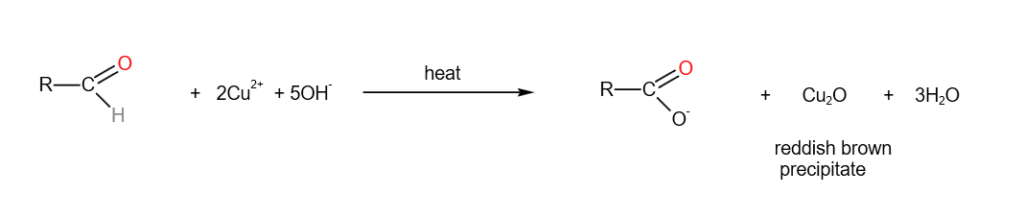
7. Iodoform Test
Iodoform test is used to test for a specific structure. We’ve discussed this in the alcohol chapter. You can refer to it here.
Organic Chemistry Reactions: Carboxylic acids and derivatives
In the chapter on carboxliyc acids and derivatives, the focus is on these functional groups: carboxylic acids, acyl halides and esters.
Carboxylic acid reactions
- Reduction
Carboxylic acids can be reduced to primary alcohols using LiAlH4 in dry ether.
RCOOH + 4[H] —–> RCH2OH + H2O
2. Condensation

3. Nucleophilic substitution
Carboxylic acids can be converted to acyl halides using one of the following:
A. HX (g), heat (where X is Cl, Br or I)
B. PX3, r.t.p (where X is Cl, Br or I)
C. PCl5 (r.t.p)
D. SOCl2 (r.t.p)
Reaction: RCOOH —> RCOX
4. Acid reactions:
A. Acid- metal
Carboxylic acids react with reactive metals such as sodium to form salt and hydrogen.
RCOOH + Na —> RCOO–Na+ + 1/2 H2
B. Acid- alkali
Carboxylic acids react with alkalis such as sodium hydroxide to form salt and water.
RCOOH + NaOH —> RCOO–Na+ + H2O
C. Acid- carbonate
Carboxylic acids react with carbonates such as sodium carbonate to form salt, water and carbon dioxide.
2RCOOH + Na2CO3 —> 2RCOO–Na+ + 2H2O + 2CO2
5. Oxidation for some carboxylic acids
Carboxylic acids are generally quite stable to oxidation (i.e. they do not undergo oxidation easily). However, there are some carboxylic acids that can be more easily oxidised.
Here’s some reactions for selected carboxylic acids that you’ll need to know for H2 A Level Chemistry:
Oxidation of methanoic acid:
Methanoic acid can be oxidised to carbon dioxide and water using
(i) Tollen’s reagent,
(ii) Fehling’s reagent,
(iii)KMnO4 (aq), H2SO4(aq), heat
(iv) K2Cr2O7 (aq), H2SO4(aq), heat
HCOOH + [O] —> CO2 + H2O
Oxidation of ethanedioic acid
ethanedioic acid can be oxidised to carbon dioxide and water using KMnO4 (aq)/ H2SO4(aq), heat.
HOOC-COOH + [O] —> 2CO2 + H2O
Acyl halide reactions:
- Hydrolysis
Acyl halides react with water to form carboxylic acid and HX at room temperature.
R-COX + H2O —-> RCOOH + HX
2. Acylation

3. Reaction with ammonia and amines
Acyl halides react with ammonia to form amides.
R-COX + NH3 —> R-CONH2 + HX
Similarly, acyl halides can react with amines to form amides.
R-COX + R’NH2 —> R-CONHR’+ HX
Esters reactions
- Acid hydrolysis
Esters undergo acidic hydrolysis to form carboxylic acid and alcohol.
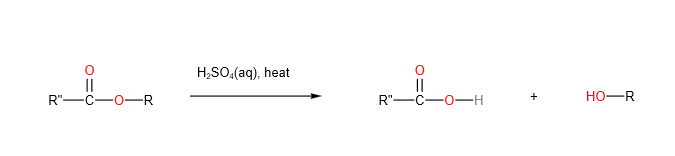
2 .Alkaline hydrolysis
Esters also undergo alkaline hydrolysis to form the salt of the carboxylic acid and alcohol. If you have phenol instead of alcohol being formed, then the phenoxide ion will be formed instead, since phenols are acidic enough to react with alkalis.
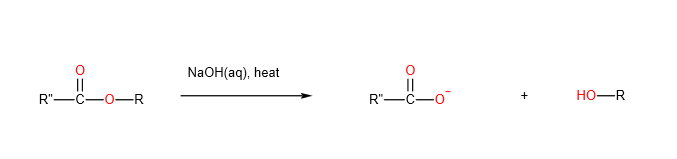
Organic Chemistry Reactions: Nitrogen Compounds
Reaction of amines:
- Amines react with halogenoalkanes.

2. Amines react with acyl halides to form amides. (Note: Only primary and secondary amines can react with acyl halides. Tertiary amines are not able to react with acyl halides.)
R-COX + R’NH2 —> R-CONHR’+ HX
3. Acid- base reaction
Amines are bases, and react with acids.
E.g. RNH2 + HCl –> RNH3+Cl–
Reactions of amides:
- Reduction
Amides are reduced by LiAlH4 in dry ether to form amines.
RCONH2 —–> RCH2NH2
2. Acid hydrolysis
Amides undergo acid hydrolysis to form carboxylic acid and the salt of the amine.
RCONHR” —-> RCOOH + NH2+R”
3. Alkaline hydrolysis
Amides undergo alkaline hydrolysis to form the salt of the carboxylic acid and amine.
RCONHR” —-> RCOO – + NH2R”
Reactions of nitriles
- Reduction of nitriles (-CN)
Nitrile groups can be reduced to form amines using LiAlH4 in dry ether:
RCN —> RCH2NH2
2. Acid hydrolysis of -CN group
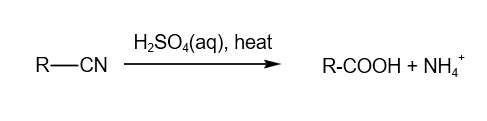
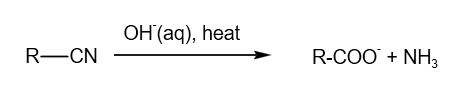
3. Alkaline hydrolysis of -CN group
Formation of phenylamine
Phenylamine can be formed from nitrobenzene by reduction.
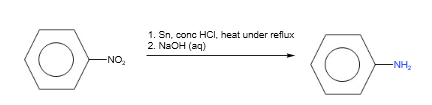
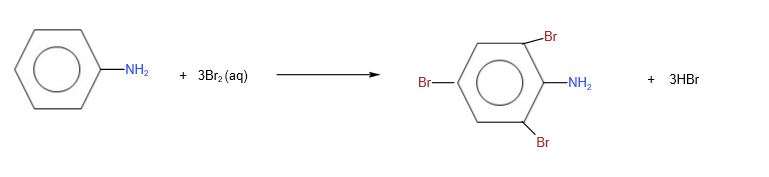
Reaction of benzene phenylamine
Electrophilic substitution of the benzene ring of phenylamine
In phenylamine, the lone pair electrons on O in -OH group delocalises into the benzene ring, making the benzene ring more electron rich and as such more susceptible to electrophilic attack.
Organic Chemistry Reactions tested in A Level H2 Chemistry
Knowing the reactions is just the first step for doing well in your A Level H2 Chemistry Organic Chemistry exam. You’ll need to know how to apply, determine structures from given reactions, distinguish various organic compounds from one another and more. But, without knowing these reactions well, it’s going to be difficult to apply these questions.
Hence, the first step to learn A Level H2 Organic Chemistry is to learn the reactions well.
Related Post
Learn H2 Chemistry On- Demand
Related Books
Organic Chemistry: An Essential Summary for H2 A Level Chemistry Students
Revision
Reactions
- Organic Chemistry Reactions Tested in H2 A Level Chemistry
- Organic Chemistry Reaction Mechanisms
- Organic Chemistry Concept Maps Part 1
- Organic Chemistry Concept Maps Part 2
- Structural Elucidation Questions

