Explaining and comparing the basicity of amines and amides are often tested in H2 A Level Chemistry exams. In this post, let’s look at :
- what makes a compound basic
- why are amines basic
- factors affecting basicity
- comparing basicity of amines and phenylamines
- why are amides neutral
What makes a compound basic?
There are many definitions of bases. If we use the Bronsted Lowry theory, then a base is a proton acceptor. If a compound accepts a proton (H+) , it will be acting as a base.
Let’s use ammonia as an example.
NH3 + H2O ⇌ NH4+ + OH–
In the equation above, ammonia (NH3) accepts a proton (H+) from water to form NH4+ . Since it accepts a proton, it is a base.
Why are amines basic?
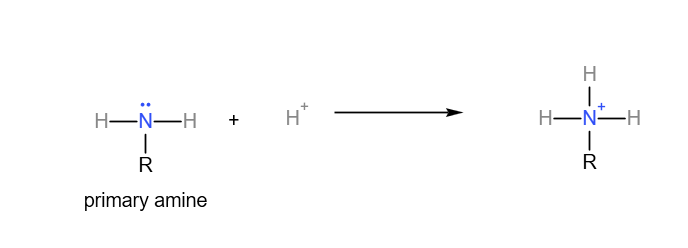
The lone pair electron on nitrogen in an amine is able to form a dative covalent bond with a proton. Since it accepts a proton, amines can act as bases.
Primary amine accepting a proton:
Secondary amine accepting a proton:

Tertiary amine accepting a proton:

Note: Every time an amine accepts a proton, the number of H bonded to nitrogen increases by 1, and the charge also increases by 1.
Factors affecting basicity of amines
The basicity of amines is determined by its availability of the lone pair electron on the nitrogen atom to accept a proton. The higher the charge density, the greater the availability of this lone pair of electrons, and hence the more basic the amine is.
Since alkyl group (or R groups) are electron donating, tertiary amines are generally more basic than secondary amine, and secondary amines are more basic and primary amines.
Comparing basicity of aliphatic amines and phenylamines
Aliphatic amines are more basic than phenylamines.
In phenylamine, the lone pair electron on nitrogen can delocalise into the benzene ring. This reduces the electron density of the lone pair electron on nitrogen, and hence its availability to form a dative bond with a proton.

Why are amides neutral?
We have talked about amines for most of this post. In this post, let’s talk about why amides are neutral.

The lone pair of electrons on nitrogen can delocalise into the C=O group, making this lone pair of electrons on nitrogen not available for dative bonding with a proton. Hence, amides are neutral.

p/s: From past A Level exams, “why amides are neutral” is a common question tested in H2 A Level Chemistry.
Explanation Questions for Organic Chemistry
In organic chemistry, explanation questions are very common. Asking students to explain the different basicity of amines and amides (i.e. nitrogen compounds) is one of them. There are also other types of explanation questions that are tested.
This entire post is on basicity of organic compounds. If you are also interested in acidity of organic compounds, check out this post here.
Related Post
Learn H2 Chemistry On- Demand
Related Books
Organic Chemistry: An Essential Summary for H2 A Level Chemistry Study
Revision
Reactions
- Organic Chemistry Reactions Tested in H2 A Level Chemistry
- Organic Chemistry Reaction Mechanisms
- Organic Chemistry Concept Maps Part 1
- Organic Chemistry Concept Maps Part 2
- Structural Elucidation Questions