Acidity of organic compounds, specifically alcohols, phenols and carboxylic acid, and explanation of their relative acidity is part of the H2 A Level Chemistry syllabus. In this post, let’s look at:
- Why are some organic compounds acidic?
- Relative acidity of organic compounds i.e. alcohols, phenols, carboxylic acids and benzoic acid, and how to explain the differences in acidity.
- Reaction of alcohols, phenols and carboxylic acids with metals, alkalis and carbonates
Why are some organic compounds acidic?
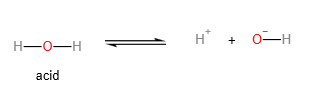
An acid is a proton donor (according to Bronsted Lowry theory). The more readily the acid loses the proton, the more acidic it is. In order for the acid to lose the proton more readily, the anion (or salt of the acid) formed should be as stable as possible. The lower the concentration of the negative charge on the anion, the more stable it is, and therefore, the more acidic the acid.
Here are some examples of Bronsted acids:
- Water as an acid:
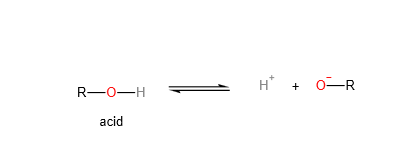
2. Alcohol as an acid:
3. Phenol as an acid

4. Carboxylic acid as an acid:

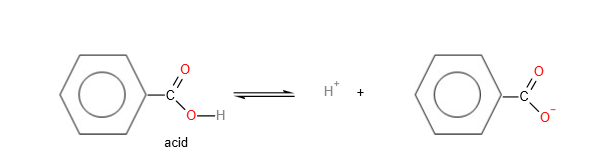
5. Benzoic acid as an acid:
Comparing and explaining acidity of organic compounds
The relative acidity of the compounds are as follows:
benzoic acid (most acidic) > carboxylic acid > phenol > water > alcohol
Water is more acidic than alcohol
Alcohol is less acidic than water. The alkyl group is electron donating compared to the -H group. This electron donating group will increase the electron density of the negative charge on the oxygen atom, destabilising the anion. Hence, alcohol is less acidic than water.
Phenol is more acidic than alcohol
Phenols are more acidic than alcohols. The negative charge on oxygen in the phenoxide ion can delocalise into the benzene ring, reducing the charge density, and hence increasing the stability of the phenoxide ion.

Carboxylic acid is more acidic than phenol
The salt of the carboxylic acid or the carboxylate anion or RCOO-, has 2 equivalent resonance structures which allows the delocalisation of the negative charge over 2 oxygen atom. This results in greater stability of carboxylate anion compared to the phenoxide anion. Hence, carboxylic acid is more acidic than phenol
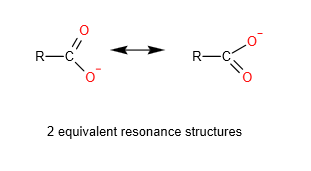
Benzoic acids are more acidic than aliphatic carboxylic acids
Both the salts of the benzoic acid and the salts of carboxylic acids have the 2 equivalent resonance structure which will enhance stability of the salt, and hence increase acidity. However, for the salt of the benzoic acid, the negative charge on the oxygen atom can also delocalise into the benzene ring, further enhancing stability of the anion. Hence, benzoic acids are more acidic than aliphatic carboxylic acids.
How acidity of organic compounds affect reactions.
Now that we have learnt the relative acidities of alcohols, phenols and carboxylic acids, let’s look at their reactions with metals, alkalis and carbonates.
| Reacts with sodium? | Reacts with sodium hydroxide? | Reacts with sodium carbonate? | |
| alcohol | Yes | No | No |
| phenol | Yes | Yes | No |
| carboxylic acid (including benzoic acid) | Yes | Yes | Yes |
These are part of the list of reactions that you should know for A Level Organic chemistry. For full list of reactions, see this post.
Related Post
Learn H2 Chemistry On- Demand
Related Books
Organic Chemistry: An Essential Summary for H2 A Level Chemistry Study
Revision
Reactions
- Organic Chemistry Reactions Tested in H2 A Level Chemistry
- Organic Chemistry Reaction Mechanisms
- Organic Chemistry Concept Maps Part 1
- Organic Chemistry Concept Maps Part 2
- Structural Elucidation Questions

