In this post, we’ll look at the reactivity series of metals (tested in the O Level exam), and then have a look at some of the reactions of metals.
Reactivity Series
A reactivity series classify the metals according to their reactivity. Here is the reactivity series for common metals. The higher the metal is in the reactivity series, the more reactive it is.
Potassium (K) <—– Most reactive
Sodium (Na)
Calcium (Ca)
Aluminium (Al)
Zinc (Zn)
Iron (Fe)
Lead (Pb)
Hydrogen (H)
Copper (Cu)
Silver (Ag)
Gold (Au) <— least reactive
Reaction of Metals
In the O Level Chemistry exam, you are tested on the following reactions of metals:
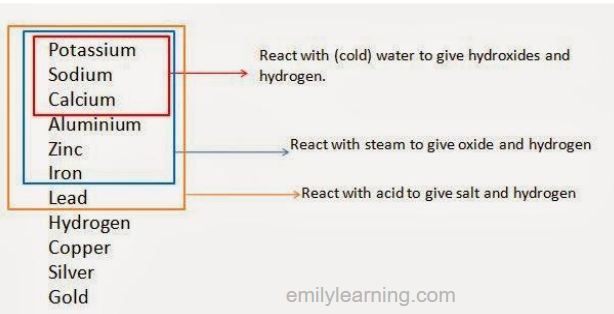
- Reaction of metals with water
- Reaction of metals with steam (i.e. water in gaseous state)
- Reaction of metal with acid
The diagram below shows the reaction of common metals:
Reaction of Metal Compounds
In the metal chapter, you are expected to know these reactions of metal compounds:
Extraction of metal
Extraction of metal refers to obtaining the metal from its ore (i.e. metal compound). The more reactive the metal, the harder it is to extract it.
Reactive metals can only be extracted from their ore by electrolysis (which is very expensive). Less reactive metal can be extracted from their ore by heating with reducing agent (which is generally less expensive that electrolysis).
The diagram below shows a summary of how common metals are extracted:

Thermal decomposition of metal carbonates (i.e. heating the metal carbonates)
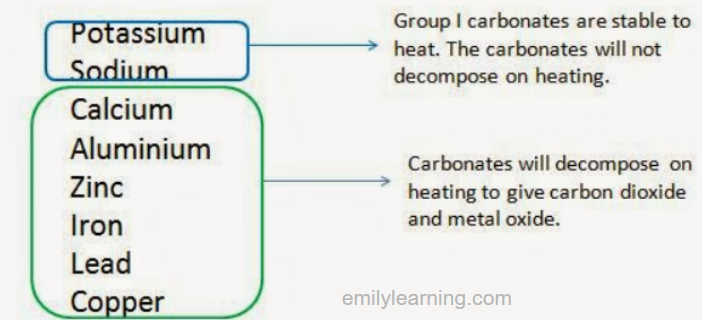
The carbonates of less reactive metals undergo thermal decomposition more readily.
Group I metals carbonates are very stable to heat. Carbonates like potassium carbonate and sodium carbonate will not decompose on heating.
Carbonates of less reactive metals (e.g. calcium carbonate, aluminium carbonate, etc.) undergo thermal decomposition to give metal oxide and carbon dioxide.
The diagram below summarizes the effect of heating the carbonates of the following metals:
Displacement reactions of metals
A more reactive metal will displace a less reactive metal.
Example questions of displacement reactions of metals
Example 1: Will zinc react with copper(II) sulfate?
Solution: Yes
Zinc is more reactive than copper, hence, zinc will displace copper from copper(II) sulfate. The products of this reaction are zinc sulfate and copper.
Example 2: Will iron react with magnesium chloride?
Solution: No
Iron is less reactive than magnesium. Hence, iron will not displace magnesium from magnesium chloride.
Learn O Level Chemistry topics on-demand
Want this topic and other topics tested in the O Level Chemistry (Pure) syllabus? Check out our On-demand chemistry courses written based on the Singapore O Level Chemistry syllabus.
