In this post, let’s talk about drawing dot- and- cross diagrams of covalent molecules.
A covalent bond is a chemical bond formed by sharing of electron pairs between atoms.
Why do non- metals form covalent compounds
In O Level Chemistry, students learn that generally, non- metals and non- metals share pair(s) of electrons to form covalent bond(s). Think of it as when you have non-metals and non-metals, which are usually in Groups IV – VII. In these groups, it is easier to gain stable electronic configuration by sharing electrons, or gaining electrons, rather than losing them. If you have 2 atoms that want to gain electrons in order to achieve the stable octet configuration, then it will be easier for the atoms to share and achieve 8 than to lose or gain electrons. That’s why, non- metals and non- metals form covalent compounds.
In short, non- metals and non- metals form covalent bonds with one another as it’s easier for them to achieve stable electronic configuration this way.
Drawing covalent bonding in molecules using dot- and- cross diagrams
Now that we know that non- metals and non- metals form covalent bonds, let’s look at how are these covalent bonds represented. A method to represent the covalent bonds in a diagram is by drawing dot- and- cross diagrams. In these diagrams, we use circles to represent the electron shells. As covalent bonds are formed by sharing of electrons between atoms, we draw overlapping circles to show the overlapping of the electron shells, and draw in pairs of dots and crosses to show the sharing of electrons.
Covalent molecules examples
Let’s look at how to illustrate the covalent bonds using dot- and- cross diagrams.
I have divided the examples into two groups:
- Diatomic molecules – such molecules are formed from 2 atoms covalently bonded to each other
- Polyatomic molecules – such molecules are formed from more than 2 atoms
In all the examples below, only the outermost electrons are shown.
Click on the link below to go directly to the molecule.
Drawing dot- and- cross diagrams of Diatomic molecules
- Hydrogen (H2)
- chlorine (Cl2)
- fluorine (F2)
- bromine (Br2)
- oxygen (O2)
- nitrogen (N2)
- hydrogen chloride (HCl)
Drawing dot- and- cross diagrams of Polyatomic molecules
Diatomic Molecules
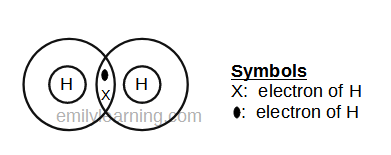
Dot- and- cross diagram of covalent molecule (hydrogen) H2
Hydrogen is in the first period of the periodic table. This means that it can only hold a maximum of 2 electrons. Instead of achieving stable octet configuration, hydrogen achieves stable duplet configuration.
In the molecule of hydrogen, each hydrogen atom donates one electron to the covalent bonding, and both hydrogen atoms will have the stable duplet configuration.
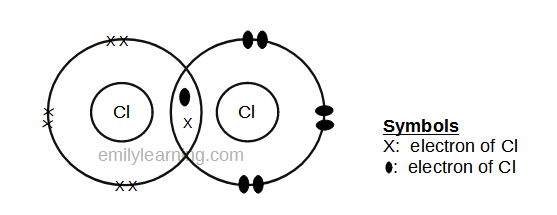
Dot- and- cross diagram of covalent molecule (chlorine) Cl2
Chlorine is in the third period of the periodic table. Unlike hydrogen, it will tend to achieve stable octet configuration. Hence, we’ll look at having 8 valence electrons for chlorine. Since chlorine has 7 valence electrons (as it is in Group VII), it needs just 1 more electron to achieve stable octet configuration. This means that one chlorine atom will share 1 electron with another chlorine atom and both will achieve stable octet configuration.
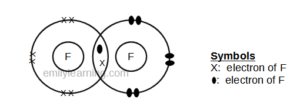
Dot- and- cross diagram of covalent molecule (fluorine) F2
Fluorine is in the second period of the periodic table, and is the first element in Group VII. It will achieve stable octet configuration in fluorine molecule. Let chlorine, fluorine has 7 valence electrons (as it is in Group VII), it needs just 1 more electron to achieve stable octet configuration. This means that one fluorine atom will share 1 electron with another fluorine atom and both will achieve stable octet configuration.
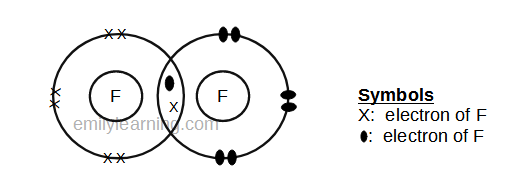
Since we are showing only the outermost electrons, you may be wondering if the dot- and- cross diagram of both fluorine and chlorine are the same. Well, when you draw them separately, the dot- and- cross diagrams of the two look similar. However, when you put the diagrams side by side, then the fluorine molecule should be smaller than the chlorine molecule. This is because being period 2, the outermost shell of fluorine is in the second principal quantum shell, while being in period 3, the outermost shell of chlorine is in the third principal quantum shell. The larger the principal quantum shell, the larger the size of the orbitals.

Dot- and- cross diagram of covalent molecule (bromine) Br2
Bromine is in the same group as fluorine and chlorine — Group VII of the periodic table. Hence, just like other elements in group VII of the periodic table, a molecule of bromine (or Br2 ) is formed by the sharing of a pair of electrons between 2 bromine atoms. To form such a bond, each bromine atom donates 1 electron for the covalent bond, and the end result is both atoms in the molecule achieve stable octet configuration.
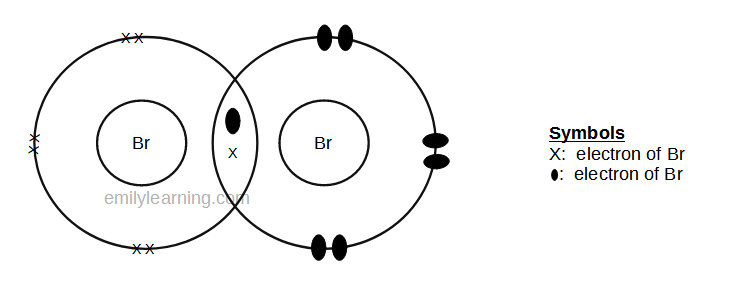
The dot-and- cross diagram of bromine will look like that of fluorine and chlorine. Only that bromine should be the biggest, followed by chlorine and then chlorine.
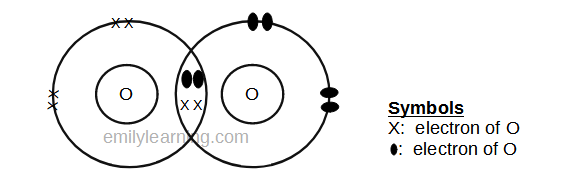
Dot- and- cross diagram of covalent molecule (oxygen) O2
Oxygen is in Group VI of the periodic table. This means that an atom of oxygen has 6 valence electrons. In order to achieve stable octet configuration, oxygen needs 8 valence electrons, in order words, 2 more electrons.
To achieve stable octet configuration, each atom of oxygen donates 2 electrons to the covalent bond to form oxygen molecules. By sharing 2 electrons, both oxygen in the oxygen molecule now have a stable octet configuration.
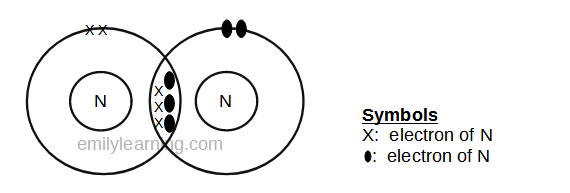
Dot- and- cross diagram of covalent molecule (nitrogen) N2
Nitrogen is in Group V of the periodic table. This means that an atom of nitrogen has 5 valence electrons. In order to achieve stable octet configuration, nitrogen needs 5 valence electrons, in order words, 3 more electrons.
To achieve stable octet configuration, each atom of nitrogen donates 3 electrons to the covalent bond to form nitrogen molecules. By sharing 3 electrons, both nitrogen in the nitrogen molecule now have a stable octet configuration.
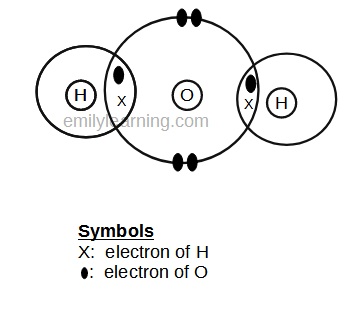
Dot- and- cross diagram of covalent molecule hydrogen chloride (HCl)
To draw hydrogen chloride, let’s look at the electronic configuration of the hydrogen and chlorine atom first.
In hydrogen atom, it only has 1 electron. Since hydrogen is in period 1, it achieves stable duplet structure rather than octet. To achieve duplet structure, it needs 2 electrons in its valence shell. This means, hydrogen needs 1 more electrons. In other words, it needs to share 1 more electrons.
For chlorine, it is in group VII, and has 7 valence electrons. It wants to achieve stable octet configuration. To do so, it will need 1 more electron. In other words, it will like to shar 1 electron.
Since both chlorine and hydrogen would like to share 1 electron to achieve stable octet and duplet configuration respectively, they will form a covalent bond with each other by sharing just 1 electron.

Polyatomic Molecules
Dot- and- cross diagram of covalent molecule water (H2O)
Let’s look at drawing the dot-and-cross diagram of water.
Oxygen is in group VI of the periodic table. With 6 valence electrons, it needs 2 more electrons. So it will share 2 electrons to achieve stable octet configuration. It can share the 2 electrons with 1 atom (and form 1 double bond) or with 2 atoms (and form 2 single bonds).
Hydrogen has 1 valence electron, and will need 1 more electron to achieve stable duplet configuration. So it will form 1 single bond with another atom.
As such, oxygen will be directly bonded to the 2 hydrogens.
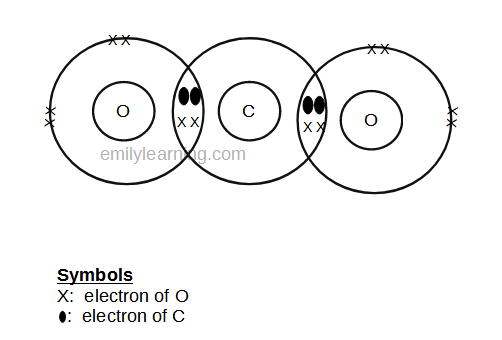
Dot- and- cross diagram of covalent molecule carbon dioxide (CO2)
Let’s look at drawing the dot-and-cross diagram of carbon dioxide.
Oxygen is in group VI of the periodic table. With 6 valence electrons, it needs 2 more electrons. So it will share 2 electrons to achieve stable octet configuration. It can share the 2 electrons with 1 atom (and form 1 double bond) or with 2 atoms (and form 2 single bonds).
Carbon is in group IV of the periodic table. With valence 4 electrons, it needs 4 more electrons. So it will share 4 electrons to achieve stable octet configuration. Since in a molecule of carbon dioxide, there are just 2 other oxygen apart from carbon, this means that these 4 electrons will be shared with oxygen. Since each oxygen needs 2, carbon will share 2 electrons with each oxygen.
Dot- and- cross diagram of covalent molecule ammonia (NH3)
Let’s look at drawing the dot-and-cross diagram of ammonia.
Nitrogen is in group V of the periodic table. With 5 valence electrons, it needs 3 more electrons. So it will share 3 electrons to achieve stable octet configuration.
Hydrogen has 1 valence electron, and will need 1 more electron to achieve stable duplet configuration. So it will form 1 single bond with another atom. Since there are 3 hydrogen atoms in ammonia, 1 nitrogen atom will form single bonds with 3 hydrogen atoms to form a molecule of ammonia.

Dot- and- cross diagram of covalent molecule methane (CH4)
Let’s look at drawing the dot-and-cross diagram of methane.
Carbon is in group IV of the periodic table. With valence 4 electrons, it needs 4 more electrons. So it will share 4 electrons to achieve stable octet configuration. Since in a molecule of methane, there are just 4 other hydrogen atoms apart from carbon, this means that these 4 electrons of carbon will be shared with hydrogen.

Dot- and- cross diagram of covalent molecule ethene (C2H4)
Let’s look at drawing the dot-and-cross diagram of ethene.
If you have learnt the alkene chapter in Organic Chemistry chapter, then you would have learnt that ethene, being an alkene has a C=C. In this case, the 2 carbons in ethene form double bonds with each other. Since carbon is in group IV of the periodic table. With valence 4 electrons, it needs 4 more electrons. Since carbon has used 2 of the valence electrons in the C=C, it needs to form 2 more bonds, and these will be formed with hydrogen. Each carbon forms 2 bonds with 2 hydrogens.

Dot- and- cross diagram of covalent molecule hydrogen peroxide (H2O2 )
Let’s look at drawing the dot-and-cross diagram of hydrogen peroxide.
Oxygen is in group VI of the periodic table. With 6 valence electrons, it needs 2 more electrons. So it will share 2 electrons to achieve stable octet configuration. In hydrogen peroxide, each oxygen atom forms a single bond with another oxygen atom, and a hydrogen atom.

How to be good at drawing dot- and- cross diagrams of covalent molecules?
The best way to be good at drawing dot- and- cross diagrams is to see more and practice more.
After looking at all these examples above, practice drawing them without looking at the diagrams. It’s only through doing (practicing) that you’ll truly learn and remember.
Learn O Level Chemistry topics on-demand
Want this topic and other topics tested in the O Level Chemistry (Pure) syllabus? Check out our On-demand chemistry courses written based on the Singapore O Level Chemistry syllabus.