In this post, let’s look at how to write electronic configuration of atoms and ions the O Level Chemistry way.
Here are the sections we are covering in this post. Click on the section you are interested to go there directly:
- what is electronic configuration
- how to write electronic configuration the O level way
- Writing the electronic configuration of atoms
- Worked Example 1: Writing the electronic configuration of chlorine atom
- Worked Example 2: Writing the electronic configuration of sodium atom
- Worked Example 3: Writing the electronic configuration of calcium atom
- Writing the electronic configuration of ions
- Worked Example 4: Writing the electronic configuration of chlorine ion
- Worked Example 5: Writing the electronic configuration of sodium ion
- Worked Example 6: Writing the electronic configuration of calcium ion
- What’s next?
What is electronic configuration?
The electronic configuration shows how the electrons are distributed.
In O Level Chemistry, students are only expected to learn about electron shells (and not subshells). Hence, we write the distribution of electrons according to the shells.
Electrons fill the shells closest to the nucleus first. The ones with the lowest principal quantum number is first being filled. The maximum number of electrons that can be in a principal quantum shell is 2n2. Base on this, the maximum number of electrons in the first shell is 2 (since 2(1)2 = 2), the maximum number of electrons in the second shell is 8 (since 2(2)2 = 8), the maximum number of electrons in the third shell is 18 (since 2(3)2 = 18), and so on. For O Level students, they learn that even for the third shell, they will only fill 8 (for their syllabus). The main reason is because some of the subshells in the 3rd principal quantum shell are at a higher energy level than the 4th principal quantum shell, and we will fill some of the electrons in the 4th principal quantum shell before moving back to the third principal quantum shell (you will learn this in H2 A Level Chemistry). If this confuses you, then just remember this:
- fill a maximum of 2 electrons in the first shell
- fill a maximum of 8 electrons in the second shell
- fill 8 electrons in the third shell then move on to the fourth shell
The O Level syllabus will not involve writing electronic configuration involving more than 8 electrons in the 3rd principal quantum shell (that will be for transition metals). Also, you won’t be expected to go beyond 4 shells in the O Level syllabus.
How to write the electronic configuration the O level way
To write the electronic configuration the O level way, you’ll write them in brackets. (2,8,1) is an example of an electronic configuration. We write the number of electrons in each principal quantum shell, starting from the smallest one on the left. Number of each principal quantum shell is separated from the next by a comma.
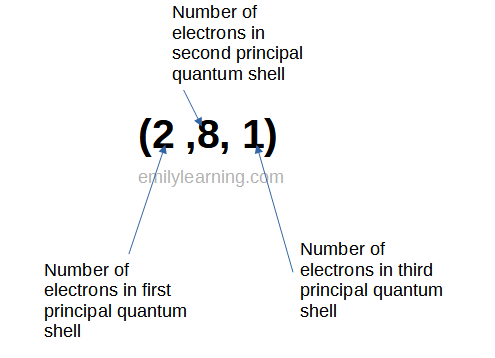
Writing the electronic configuration of atoms
Next, let’s look at how to write the electronic configuration of atoms.
Here’s a break down of the steps to do so:
- Refer to the periodic table to determine atomic number (or proton number)
- Determine number of electrons by using this concept: Number of protons = number of electrons for atoms.
- Put 2 electrons into the first shell, If there are electrons left, put into second shell, and then third shell and so on until all the electrons have been used up.
Worked Example 1: Writing the electronic configuration of chlorine atom
Let’s write the electronic configuration of chlorine atom together in this example.
Step 1: Referring to the periodic table, chlorine has a proton number of 17.
Step 2: The number of electrons of a chlorine atom = number of protons of a chlorine atom = 17
Step 3: Now that we know that chlorine atom has 17 electrons, let’s put them into shells.
The first shell will have 2 electrons. After this, we have 15 electrons left.
Let’s put 8 electrons into the 2nd shell. with that, we have7 electrons left.
Let’s put all the remaining 7 electrons into the third shell.
There, we have the electronic configuration of chlorine: (2,8,7).
Worked Example 2: Writing the electronic configuration of sodium atom
Let’s write the electronic configuration of sodium atom together in this example.
Step 1: Referring to the periodic table, sodium has a proton number of 11.
Step 2: The number of electrons of a sodium atom = number of protons of a sodium atom = 11
Step 3: Now that we know that sodium atom has 11 electrons, let’s put them into shells.
The first shell will have 2 electrons. After this, we have 9 electrons left.
Let’s put 8 electrons into the 2nd shell. with that, we have 1 electron left.
Let’s put all the remaining 1 electron into the third shell.
There, we have the electronic configuration of sodium: (2,8,1).
Worked Example 3: Writing the electronic configuration of calcium atom
Let’s write the electronic configuration of calcium atom together in this example.
Step 1: Referring to the periodic table, calcium has a proton number of 20.
Step 2: The number of electrons of a calcium atom = number of protons of a calcium atom = 20
Step 3: Now that we know that calcium atom has 20 electrons, let’s put them into shells.
The first shell will have 2 electrons. After this, we have 18 electrons left.
Let’s put 8 electrons into the 2nd shell. with that, we have 10 electron left.
Let’s put 8 electrons into the 3rd shell. (Remember for your O Level syllabus, we only fill 8 electrons into the 3rd shell. This is because in the 3rd shell, we fill 8 electrons first, then move on to fill up to 2 electrons in the 4th shell, then go back to fill the remaining 10 electrons in the 3rd shell. This is no in the O Level syllabus. You’ll learn more about this in A level Chemistry.) With that we have 2 electrons left.
These 2 electrons are placed into the 4th shell.
There, we have the electronic configuration of calcium: (2,8, 8, 2)
Writing the electronic configuration of ions
Next, let’s look at how to write the electronic configuration of ions. We know that ions are charged particles. Positively charged ions or cations are formed by the loss of electrons. This means that they have more protons than electrons. Conversely, negatively charged ions or anions are formed by gaining of electrons. This means that they have more electrons than protons.
To find the number of electrons of ions, we take number of proton (which will always remain the same) – (charge).
Here’s a break down of the steps to do so:
- Refer to the periodic table to determine atomic number (or proton number)
- Determine number of electrons by using this concept: Number of electrons in ions = proton number – (charge)
- Put 2 electrons into the first shell, If there are electrons left, put into second shell, and then third shell and so on until all the electrons have been used up.
Worked Example 4: Writing the electronic configuration of chlorine ion (Cl–)
Let’s write the electronic configuration of chlorine atom together in this example.
Step 1: Referring to the periodic table, chlorine has a proton number of 17.
Step 2: Chlorine ion has a charge of -1.
The number of electrons of a chlorine ion = proton number – charge = 17 – (-1) = 18
Step 3: Now that we know that chlorine ion has 18 electrons, let’s put them into shells.
The first shell will have 2 electrons. After this, we have 16 electrons left.
Let’s put 8 electrons into the 2nd shell. with that, we have 8 electrons left.
Let’s put all the remaining 8 electrons into the third shell.
There, we have the electronic configuration of chlorine ion, Cl– : (2,8,8).
Worked Example 5: Writing the electronic configuration of sodium ion (Na+)
Let’s write the electronic configuration of sodium atom together in this example.
Step 1: Referring to the periodic table, sodium has a proton number of 11.
Step 2: Sodium has a charge of +1. The number of electrons of a sodium ion =proton number – charge = 11-1 = 10
Step 3: Now that we know that sodium ion has 10 electrons, let’s put them into shells.
The first shell will have 2 electrons. After this, we have 8 electrons left. Let’s put the remaining 8 electrons into the second shell.
There, we have the electronic configuration of sodium ion, Na+: (2,8).
Worked Example 6: Writing the electronic configuration of calcium ion (Ca2+)
Let’s write the electronic configuration of calcium ion together in this example.
Step 1: Referring to the periodic table, calcium has a proton number of 20.
Step 2: Calcium has a charge of +2. The number of electrons of a calcium ion = proton number – charge = 20 – 2 = 18
Step 3: Now that we know that calcium ion has 18 electrons, let’s put them into shells.
The first shell will have 2 electrons. After this, we have 16 electrons left.
Let’s put 8 electrons into the 2nd shell. with that, we have 8 electron left.
Let’s put the remaining 8 electrons into the 3rd shell. (Remember for your O Level syllabus, we only fill 8 electrons into the 3rd shell. This is because in the 3rd shell, we fill 8 electrons first, then move on to fill up to 2 electrons in the 4th shell, then go back to fill the remaining 10 electrons in the 3rd shell. This is no in the O Level syllabus. You’ll learn more about this in A level Chemistry.)
There, we have the electronic configuration of calcium ion, Ca2+ : (2,8, 8)
Learn O Level Chemistry topics on-demand
Want this topic and other topics tested in the O Level Chemistry (Pure) syllabus? Check out our On-demand chemistry courses written based on the Singapore O Level Chemistry syllabus.

