Qualitative analysis is the process of identifying chemical components present in a sample by doing tests.
In this post, let’s talk about the type of changes we expect to see during qualitative analysis tests.
- Examples of observations during qualitative analysis
- Templates to describe observations during qualitative analysis
- More on qualitative analysis
Examples of observations during qualitative analysis
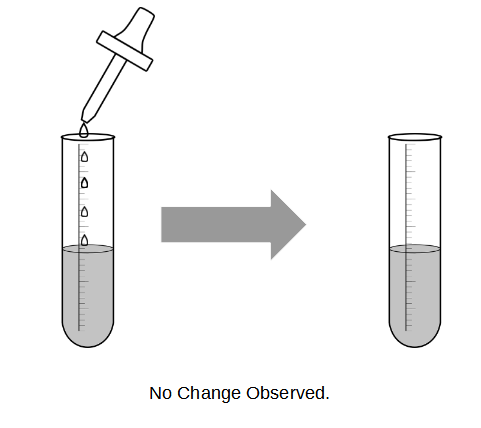
Let’s use the following as an example. Say we have a sample of solution, and we want to find out what’s present. How would you do so? There are many ways to do so. One method is via adding of chemicals, and looking out for a change. Observing a change is important. If there is no change (i.e. the solution remains the same colour, no gases evolve, etc) we have no idea whether a reaction has taken place, or we have simply just mixed the two chemicals together.
Example of no observable change in qualitative analysis test
Examples of observable changes in qualitative analysis tests



In short, we are looking for an observable change during qualitative analysis. The change could be:
- gas being produced
- change in colour of the reactants
- dissolving of a solid
- producing a solid
Note, during qualitative analysis, you could see more than one of the above change happening at the same time.
Also, do note that if there is no observable change, it doesn’t mean no reaction. It simply means no reaction that produces a change.
Templates to describe observations during qualitative analysis
Here are some templates to describe changes happening during qualitative analysis test.
| What happened | Template description |
| Nothing happens | No observable reaction |
| Solution change colour | Solution changed from (initial colour) to (final colour). |
| Change in colour of solid. | Solid changed from (initial colour) to (final colour) If you see multiple colour change, use something like this: Solid changed from (initial colour) to (colour) then (colour) and finally to (colour). |
| Gas produced from a sample containing only solid. | (colour), (smell) of gas produced, which (talk about effect on moist litmus paper), and (distinguishing test for gas). |
| Gas produced from a sample containing liquid. | Effervescence of (colour), (smell) of gas produced, which (talk about effect on moist litmus paper), and (distinguishing test for gas). |
| Solid produced after adding solutions. | (colour) precipitate produced. |
| Solid dissolves in solution. | (colour) solid dissolves in solution to give a (colour) solution. |
Some things to take note when writing observations:
- precipitate is formed from solutions.
- You only see effervescence (i.e. bubbles) if you start off with liquids. No bubbles will be seen if you only have solids, so don’t use effervescence to describe gases produced when you are heating solids!